ABOUT ASCENIVTM
Study Design: A 52-week, prospective, open-label, nonrandomized, multicenter, phase 3 study that evaluated the efficacy and safety of ASCENIV (formerly RI-002) in patients with PI (N=59). Intravenous infusions were administered at doses of 300 to 800 mg/kg every 3 or 4 weeks. There were 19 subjects with a 3-week cycle and 40 subjects with a 4-week cycle. There were 45 subjects (76%) with common variable immunodeficiency (CVID) as their primary diagnosis, followed by X linked agammaglobulinemia (10%), antibody deficiencies and "other" (7% each). The modified intent-to-treat (mITT) population included 59 subjects and was used for efficacy analysis. Primary efficacy endpoint was the demonstration of an SBI rate of <1.0 per person-year during the 52-week treatment period. Secondary efficacy endpoints included number of missed days of work/school/activity due to infection, unscheduled visits to the physician, and days hospitalized because of infection.5
HOW ASCENIV IS MADE
Learn how ASCENIV is manufactured using patented methodologies for donor screening and plasma pooling1*
ASCENIV—the only IVIG produced from blending RSV plasma with normal source plasma1*
PLASMA COLLECTION AND SCREENING
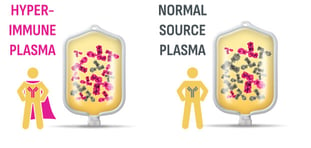
- Hyperimmune donors with RSV antibodies are identified using ADMA’s proprietary screening technology2,3
- These donors are associated with increased titers for 8 other common respiratory viruses3,4
- Plasma is collected from US FDA-licensed plasma collection centers2
TESTING

- Proprietary microneutralization assay quantifies levels of neutralizing antibodies in hyperimmune plasma donor samples2
TAILORED POOLING

ASCENIV is the only IVIG product available that is manufactured using patented methodologies for donor screening and plasma pooling*
- Normal source plasma is blended with RSV plasma to produce a tailored plasma pool derived from a minimum of 1,000 unique donors2,5
- Meets potency requirements for 21CFR6403
*ADMA BIOLOGICS PATENTS ISSUED 9,107,906 - 9,714,283 - 9,815,886.
FDA=US Food and Drug Administration; IgG=immunoglobulin G.
ASCENIV provides a broad spectrum of neutralizing IgG
antibodies against bacterial and viral pathogens and their toxins5
Expert Insights
Dr. Kelli Williams, MD, MPH shares insights from her long-term experience with ASCENIV, highlighting its benefits and the real-world impact it has had on her patients managing Primary Immunodeficiency.
Efficacy
Inside ASCENIV—efficacy to prevent serious infections5
In a 1-year, prospective, open-label, nonrandomized, multicenter, phase 3 study evaluating the efficacy and safety of ASCENIV in adult and pediatric patients with PI5:
With the proven protection of ASCENIV, reduce infection-related quality-of-life impact, so your patients can focus on what counts5
Efficacy results (PPPY) in the same 1-year study (secondary endpoints):
0
hospitalizations due to infection5
One patient from the study group was hospitalized because of a postoperative local wound infection from elective surgery5
<1
unscheduled medical visit PPPY5
24 out of 59 patients (41%) had a total of 54 unscheduled medical visits due to infections5
1.7
missed days of work/school/
activity PPPY due to infection5
23 patients (39%) had a total of 93 missed days of work/school/activity due to infections out of a total of 20,417 patient days (<0.5%)5
32.9
days of antibiotic use PPPY5
37 patients (63%) used antibiotics due to infection (includes therapeutic use)5
*SBIs were defined as a rate of <1.0 cases of bacterial pneumonia, bacteremia/septicemia, osteomyelitis/septic arthritis, visceral abscess, and bacterial meningitis per person-year.5
PPPY=per patient per year.
Quality
ADMA is committed to excellence when producing specialty biologics designed to safely and effectively treat the immunocompromised1
ADMA has created a robust, sustainable, reproducible, and controlled supply and production process for ASCENIV1
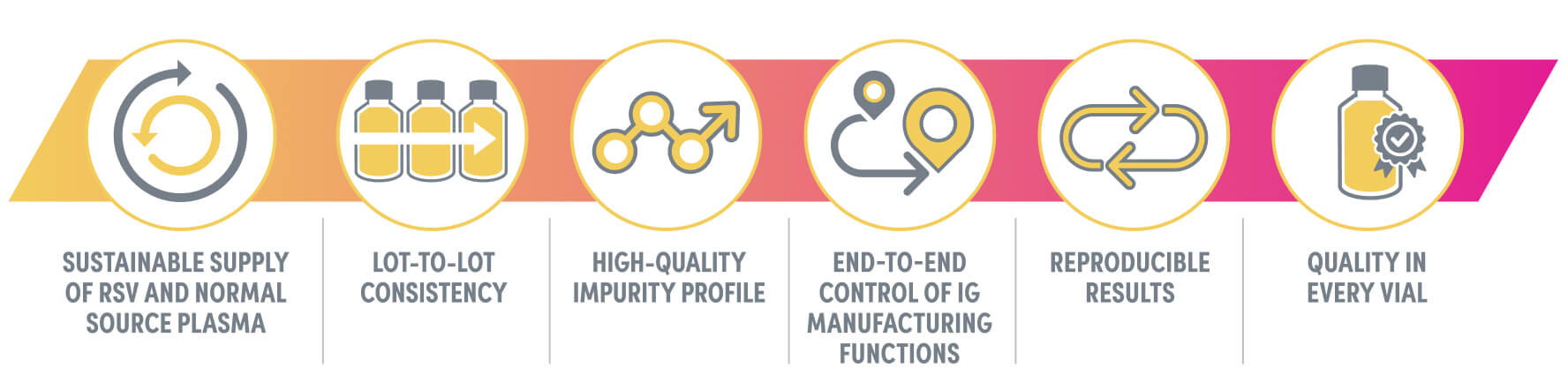
Focused on what counts: safety, quality, and efficacy
Dosing, Administration, and Storage
ASCENIV dosing, administration, and storage5
For adults and adolescents (12 to 17 years of age) with PI5
For intravenous use only
300 to 800 mg/kg every 3 to 4 weeks*
*Frequency/amount of IgG therapy may vary from patient to patient. The proper amount can be determined by monitoring clinical response.
(for first 15 minutes)
0.5 mg/kg/min
(0.005 mL/kg/min)
Increase gradually every 15 minutes up to 8 mg/kg/min (0.08 mL/kg/min)
- No apparent differences in efficacy or safety between 3- and 4-week dosing5
- The dose may be adjusted over time to achieve the desired trough levels and clinical response5
- ASCENIV dose adjustments may be required in patients who fail to maintain trough total IgG concentrations of at least 500 mg/dL with a target of 600 mg/dL. Starting with the second infusion, adjust the dose proportionally, targeting a trough of ≥600 mg/dL, based on the previous trough and the associated dose5
ASCENIV is a liquid solution containing 10% IgG (100 mg/mL) for intravenous infusion5
- Available in a single-use, non-latex, tamper-evident 5g/50 mL vial5
- Begin with an initial infusion rate of 0.5 mg/kg/min. If there are no adverse reactions, the infusion rate for subsequent infusions can be slowly increased to the maximum rate5
- Monitor patient vital signs throughout the infusion. Slow or stop the infusion if adverse reactions occur. If symptoms subside promptly, the infusion may be resumed at a slower rate that is comfortable for the patient5
- Ensure that patients with preexisting renal insufficiency are not volume-depleted. For patients judged to be at risk for renal dysfunction or thrombotic events, administer ASCENIV at the minimum infusion rate practicable, and consider discontinuation of administration if renal function deteriorates5
Storage5
- Once vial is entered, use promptly5
- Store at 2-8 °C (36-46 °F) for up to 36 months from the date of manufacture5
- Do not freeze5
- Product may be stored up to 4 weeks at ≤25 °C (77 °F). After storage at room temperature product must be used or discarded5
Safety
ASCENIV — a demonstrated safety profile5
- The total number of adverse reactions (ARs) was 158 (a rate of 0.20 ARs per infusion)5
- Fifty-eight subjects (98%) had an adverse reaction during the study. The proportion of subjects who had at least one adverse reaction was similar for both the 3- and 4-week cycles. The most common adverse reactions observed in this clinical trial were headache (22 subjects, 37%), sinusitis (16 subjects, 27%), diarrhea (14 subjects, 23%), gastroenteritis viral (13 subjects, 22%), nasopharyngitis (13 subjects, 22%), upper respiratory tract infection (13 subjects, 22%), bronchitis (12 subjects, 20%), nausea (12 subjects, 20%), and acute sinusitis (11 subjects, 19%)5
- No study drug–related serious adverse events (SAEs) were reported, although 2 SAEs (postoperative wound infection and migraine) were documented1,5
Adverse Reactions (within 72 hours after the end of an ASCENIV infusion) in ≥5% of subjects
Adverse Reactions |
Number (%) of Subjects (N=59) |
Number (%) of Infusions (N=793) |
|---|---|---|
| Headache | 14 (24) | 21 (2.6) |
| Sinusitis | 6 (10) | 7 (0.9) |
| Nausea | 5 (9) | 5 (0.6) |
| Acute sinusitis | 4 (7) | 4 (0.5) |
| Fatigue | 4 (7) | 9 (1.1) |
| Muscle spasms | 4 (7) | 4 (0.5) |
| Bronchitis | 3 (5) | 3 (0.4) |
| Diarrhea | 3 (5) | 3 (0.4) |
| Nose bleed | 3 (5) | 4 (0.5) |
| Muscle pain | 3 (5) | 5 (0.6) |
| Oropharyngeal pain | 3 (5) | 3 (0.4) |
| Pain in extremity | 3 (5) | 3 (0.4) |
| Itching | 3 (5) | 3 (0.4) |
Product Characteristics
Product characteristics5
- ASCENIV is a liquid solution containing 10% IgG (100 mg/mL) for intravenous infusion5
- The broad spectrum of neutralizing IgG antibodies against bacterial and viral pathogens and their toxins helps to avoid recurrent serious opportunistic infections5
- Purified, sterile, ready-to-use preparation5
- Clear to opalescent liquid (colorless to pale yellow)5
- Normal IgG subclass distribution5
- Formulated in water for injection containing 0.100 M-0.140 M sodium chloride, 0.20 M-0.29 M glycine, 0.15%-0.25% polysorbate 80, and pH 4.0-4.6. Contains no sucrose5
- Contains ≤200 μg/mL of immunoglobulin A5
- Mean half-life of ASCENIV5:
- 28.5 ± 4.4 days for patients on a 3-week dosing regimen
- 39.7 ± 11.6 days for patients on a 4-week dosing regimen
The only IGIV available that is manufactured using ADMA Biologics’ patented methodologies for donor screening and plasma pooling.*
Size of vial not to scale.
*Available in a single-use, non-latex, tamper-evident 5g/50 mL vial.5
Indication
ASCENIV (immune globulin intravenous, human–slra) is a 10% immune globulin liquid for intravenous injection, indicated for the treatment of primary humoral immunodeficiency (PI) in adults and adolescents (12 to 17 years of age). PI includes, but is not limited to, the humoral immune defect in congenital agammaglobulinemia, common variable immunodeficiency (CVID), X linked agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies (SCID).
Important Safety Information for ASCENIV™
WARNING: THROMBOSIS, RENAL DYSFUNCTION AND ACUTE RENAL FAILURE
- Thrombosis may occur with immune globulin (IGIV) products, including ASCENIV. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with the administration of IGIV products in predisposed patients.
- Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. ASCENIV does not contain sucrose.
- For patients at risk of thrombosis, renal dysfunction or renal failure, administer ASCENIV at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Contraindications
ASCENIV is contraindicated in:
- Patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin.
- IgA-deficiency patients with antibodies to IgA and a history of hypersensitivity.
Warnings and Precautions
Severe hypersensitivity reactions may occur with IGIV products, including ASCENIV. In case of hypersensitivity, discontinue ASCENIV infusion immediately and institute appropriate treatment. Patients with known antibodies to IgA may have a greater risk of developing potentially severe hypersensitivity and anaphylactic reactions.
Thrombosis may occur following treatment with immunoglobulin products and in the absence of known risk factors. Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity and ensure adequate hydration before administration. For patients at risk of thrombosis, administer ASCENIV at the minimum dose and infusion rate practicable. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
Acute renal dysfunction/failure, osmotic nephrosis, and death may occur upon use of human IGIV products. Ensure that patients are not volume depleted before administering ASCENIV. Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. Assess renal function, including measurement of blood urea nitrogen (BUN) and serum creatinine, before the initial infusion of ASCENIV and at appropriate intervals thereafter. Discontinue ASCENIV if renal function deteriorates. In at-risk patients, administer ASCENIV at the minimum infusion rate practicable.
Hyperproteinemia, increased serum viscosity, and hyponatremia or pseudohyponatremia may occur in patients receiving IGIV treatment, including ASCENIV. It is critical to clinically distinguish true hyponatremia from a pseudohyponatremia that is associated with or causally related to hyperproteinemia. Treatment aimed at decreasing serum free water in patients with pseudohyponatremia may lead to volume depletion, a further increase in serum viscosity, and a possible predisposition to thrombotic events.
Aseptic meningitis syndrome (AMS) may occur with IGIV treatments, including ASCENIV. AMS usually begins within several hours to 2 days following IGIV treatment. AMS may occur more frequently in association with high doses (2 g/kg) and/or rapid infusion of IGIV. Conduct a thorough neurological examination on patients exhibiting signs and symptoms of AMS, including cerebrospinal fluid (CSF) studies, to rule out other causes of meningitis.
IGIV products, including ASCENIV, may contain blood group antibodies that can act as hemolysins and induce in vivo coating of red blood cells (RBCs) with immunoglobulin, causing a positive direct antiglobulin reaction and hemolysis. Monitor patients for clinical signs and symptoms of hemolysis, including appropriate confirmatory laboratory testing.
Noncardiogenic pulmonary edema may occur with IV administered IG. Monitor patients for pulmonary adverse reactions. If suspected, perform appropriate tests for presence of anti-neutrophil antibodies in both product and patient serum. May be managed using oxygen therapy with adequate ventilatory support.
Because ASCENIV is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. All infections suspected by a physician to possibly have been transmitted by this product should be reported to ADMA Biologics at (1-800-458-4244).
After infusion of immunoglobulin, the transitory rise of the various passively transferred antibodies in the patient’s blood may yield positive serological testing results, with the potential for misleading interpretation. Passive transmission of antibodies to erythrocyte antigens (e.g., A, B, and D) may cause a positive direct or indirect antiglobulin (Coombs’) test.
Adverse Reactions
The most common adverse reactions to ASCENIV (≥5% of study subjects) were headache, sinusitis, diarrhea, gastroenteritis viral, nasopharyngitis, upper respiratory tract infection, bronchitis, and nausea.
You are encouraged to report side effects of prescription drugs to ADMA Biologics at 1-800-458-4244 or the FDA. Visit www.fda.gov/MedWatch or call 1-800-FDA-1088.
For additional safety information about ASCENIV, please see full Prescribing Information.

